Label-free Multiphoton Imaging
Two-Photon Morphometry in Muscle Health and Disease
Skeletal muscle is an archetype of SHG-susceptible tissue allowing for simultaneous imaging of label-free SHG signals originating from collagen-I (reflecting extracellular matrix arcitecture) and myosin-II (reflecting subcellular sarcomere architecture). Exploiting preferential signal scattering directionality (preferentially forward for myosin-II SHG, backward for collagen-I SHG), the signals can be optically separated and analysed in 3D using the z-sectioning actuation of the imaging stage. An example from isolated single muscle fibres is shown in the figure below demonstrating SHG-sectioning to reconstruct the three-dimensional subcellular cytoarchitecture of normal muscle cells (translating the regular striation patterns into discs) and the effect of cellular remodelling in chronic degenerative muscle disease (here: Duchenne Muscular Dystrophy; mdx mouse model), resulting in vastly distorted and tilted myofibrillar lattice geometry that explains weakness in muscle disease from a structural perspective (see also our MechaMorph system).
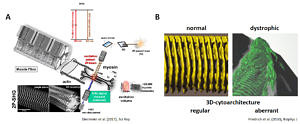 SHG imaging of label-free moysin-II signals in single muscle fibres (A) can be used to provide a 3D reconstruction of the subcellular sarcomere lattice pattern in healthy and diseased muscle. Here (B), a single fibre from a mature (10 mo) dystrophic mdx mouse is shown, demonstrating fibre splitting and subcellular derangement of the regular sarcomere lattice (Diermeier et al. 2017; Friedrich et al. 2010).
SHG imaging of label-free moysin-II signals in single muscle fibres (A) can be used to provide a 3D reconstruction of the subcellular sarcomere lattice pattern in healthy and diseased muscle. Here (B), a single fibre from a mature (10 mo) dystrophic mdx mouse is shown, demonstrating fibre splitting and subcellular derangement of the regular sarcomere lattice (Diermeier et al. 2017; Friedrich et al. 2010).
Over the years, we successively developed automated image processing tools to quantitate the geometrical disorder in sarcomere lattice in muscle diseases and sarcopenia by analysing (i) angular variability of myofibrillar orientation – as reflected by the so-called ‚cosine angle sum‘ (CAS) and (ii) parallel lattice shifts – as reflectd by so-called ‚vernier‘ densities (VD) in muscle SHG image stacks (see figure below). Those have been applied to perform quantitative morphometry of muscle cytoarchitectural changes in the due course of chronic degenerative (muscular dystrophy, R349P desminopathy) or inflammatory myopathy (critical illness myopathy), as well as ageing and muscle development. With more models and conditions being investigated, we aim to develop a morphometry database for specific remodeling patterns inherent to muscle disease fingerprints.
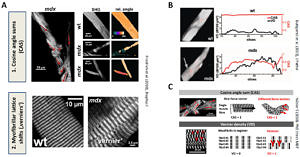 Morphological myofibrillar sarcomere patterns as revealed by SHG to quantify subcellular structural order were defined from CAS and ‚verniers‘ in muscle tissue (A). CAS and vernier densities (VD) faithfully reflect the state of myofibrillar order in healthy (wt) and disease states (mdx) (B) and can be used to quantify subcellular myofibrillar remodeling (C) (Friedrich et al. 2010; Buttgereit et al. 2013).
Morphological myofibrillar sarcomere patterns as revealed by SHG to quantify subcellular structural order were defined from CAS and ‚verniers‘ in muscle tissue (A). CAS and vernier densities (VD) faithfully reflect the state of myofibrillar order in healthy (wt) and disease states (mdx) (B) and can be used to quantify subcellular myofibrillar remodeling (C) (Friedrich et al. 2010; Buttgereit et al. 2013).
References:
- Friedrich O, Both M, Weber C, Schürmann S, et al. (2010) Microarchitecture is severely compromised but motor protein function is preserved in dystrophic mdx skeletal muscle. Biophys J. 98(4):606-16. doi: 10.1016/j.bpj.2009.11.005.
- Buttgereit A, Weber C, Garbe CS, Friedrich O (2013) From chaos to split-ups–SHG microscopy reveals a specific remodelling mechanism in ageing dystrophic muscle. J Pathol. 229(3):477-85. doi: 10.1002/path.4136.
- Diermeier S, Iberl J, Vetter K, Haug M, et al. (2017) Early signs of architectural and biomechanical failure in isolated myofibers and immortalized myoblasts from desmin-mutant knock-in mice. Sci Rep. 7(1):1391. doi: 10.1038/s41598-017-01485-x.
