PiezoGRIN: Multiphoton Imaging under high Pressures
PiezoGRIN – a Window to High Pressure Biotechnology
High pressure (HP) represents an environmental variable that attracts increasing interest in Biotechnology in terms of HP manipulation of biological processes to even HP sterilization of food. Likewise, HP affects many bio-ecosystems exposed to high ambient pressures, for instance in marine biotechnology. In order to study biosystems under elevated high pressures, sealing of recording and actuation metrologies against ambient atmospheric pressures is cumbersome and has limited the general availability of HP-technologies to study living cells and tissues. Optical, contact-free, assessment of cellular reactions under defined HP is an elegant method to bring together high hydrostatic pressure environments and readout technologies developed for atmospheric pressure conditions by separating both with optical windows that need to be designed to withstand HP forces. We recently engineered an optical pressure vessel, PiezoGRIN with the innovative aspect to incorporate tube-GRIN lenses to focus multiphoton excitation beams from an external objective into the chamber reservois containing living cells and tissue samples. This way, the GRIN lens can serve two purposes, acting as an optical element and serving as a HP barrier at the same time. The vessel is mountable to existing two-photon microscopes and is connected to an external pressure generator to create HP environments up to several hundred MPa (currently tested for 200 MPa). With this technology, we study label-free SHG signals originating from cells and tissues (e.g. skeletal muscle) under high hydrostatic pressures. Of particular interest is to delineate principal pressure limits for the pressure-induced irreversible muscle contracture (Friedrich et al. 2006; Schneidereit et al. 2018).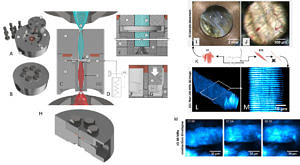 PiezoGRIN optical pressure vessel in its open stainless steel components (A) and the closed sealed vessel (B). C, side view schematics showing the symmetric GRIN lens configuration in relation to the excitation objective from below for two-photon (2p) excitation and the collection condenser lens from above. Magnifications of the sample chamber environment (E-G) show the pressure force distribution lines within the chamber connected to the external pressure hose and generator (D). (H), side view of the cut-open lower hull of the vessel depicting the fitting for the GRIN lens basin (upper inlet) and the large angle drilled hull opening to enable immersion objective optical connectivity using high NA lenses from below. PiezoGRIN optical chamber in action showing muscle single fiber and whole tissue morphology at hydrostatic pressure. K, illustration of extraction of murine musculi interossei (IO) and extensor digitorum longus (edl, EDL) as well as fixing of edl single fibers. I, immersed IO tissue in open chamber with background light coming through the lens in the center and discernible copper seal in the image perimeter. J, Bright field image of IO tissue in closed chamber showing muscle fibers and blood vessels in a field of view of 0.5 mm. L, 3D image stack (115x115x43 µm) of unstained fixed EDL single fiber pressurized at 200 MPa, obtained by recording transmitted SHG signal of 2p-excitation. M, cropped slice from within stack (L) is showing SHG signal of A-bands of the intracellular sarcomere structures from myofibrils. M, time-lapse (mm:ss) SHG images of live IO muscle in calcium free internal solution contracting after compression to 30 MPa. The compression phase concludes at t = 1 min, contracture of the fiber occurs just before the 2 minute mark (Schneidereit et al. 2018).
PiezoGRIN optical pressure vessel in its open stainless steel components (A) and the closed sealed vessel (B). C, side view schematics showing the symmetric GRIN lens configuration in relation to the excitation objective from below for two-photon (2p) excitation and the collection condenser lens from above. Magnifications of the sample chamber environment (E-G) show the pressure force distribution lines within the chamber connected to the external pressure hose and generator (D). (H), side view of the cut-open lower hull of the vessel depicting the fitting for the GRIN lens basin (upper inlet) and the large angle drilled hull opening to enable immersion objective optical connectivity using high NA lenses from below. PiezoGRIN optical chamber in action showing muscle single fiber and whole tissue morphology at hydrostatic pressure. K, illustration of extraction of murine musculi interossei (IO) and extensor digitorum longus (edl, EDL) as well as fixing of edl single fibers. I, immersed IO tissue in open chamber with background light coming through the lens in the center and discernible copper seal in the image perimeter. J, Bright field image of IO tissue in closed chamber showing muscle fibers and blood vessels in a field of view of 0.5 mm. L, 3D image stack (115x115x43 µm) of unstained fixed EDL single fiber pressurized at 200 MPa, obtained by recording transmitted SHG signal of 2p-excitation. M, cropped slice from within stack (L) is showing SHG signal of A-bands of the intracellular sarcomere structures from myofibrils. M, time-lapse (mm:ss) SHG images of live IO muscle in calcium free internal solution contracting after compression to 30 MPa. The compression phase concludes at t = 1 min, contracture of the fiber occurs just before the 2 minute mark (Schneidereit et al. 2018).
References:
- Schneidereit S, Schürmann S, Friedrich O (2018) PiezoGRIN: A High-Pressure Chamber Incorporating GRIN Lenses for High-Resolution 3D-Microscopy of living Cells and Tissues. Adv Sci. 6(4):1801453. doi: 10.1002/advs.201801453.
- Friedrich O, Wegner FV, Hartmann M, Frey B,et al. (2006) ‚In situ‘ high pressure confocal Ca(2+)-fluorescence microscopy in skeletal muscle: a new method to study pressure limits in mammalian cells. Undersea Hyperb Med. 33(3):181-95.
