The MyoRobot
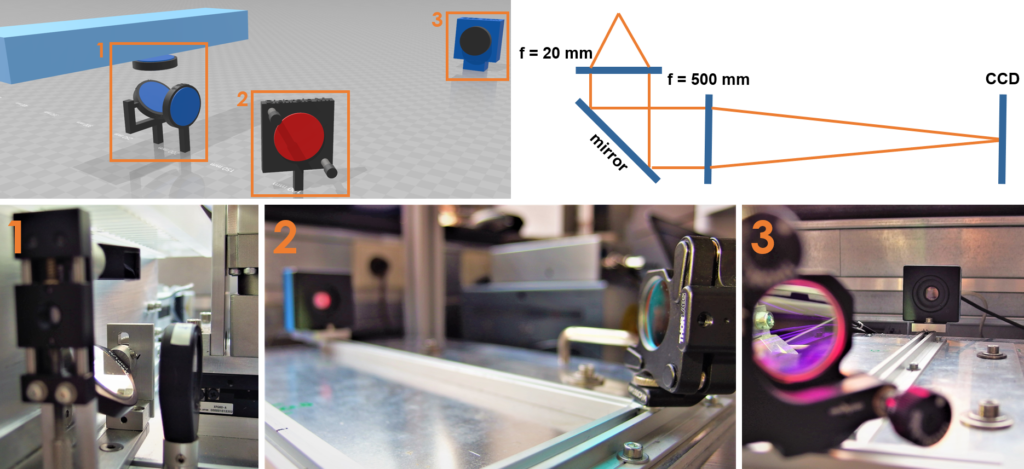
Optical Morphometry Module for the MyoRobot
Normalization of forces to fiber cross-sectional area (i.e. specific force, given in (N/m²) or (Pa)) reduces large scattering of force values among individual fibers due to fluctuations in fiber diameter. It also renders results more comparable across various organ scales (whole muscle, fiber bundles, single fibers) and among laboratories. Furthermore, control over sarcomere length ensures similar sarcomere extension in stress-strain relations which increases comparability and robustness. Therefore, we equipped the MyoRobot with an inbuilt microscope optics that produces a ~ 24x enlarged image of a single muscle fiber onto a CCD sensor. Magnification and resolution were confirmed to visualize and resolve sarcomeres above ~1.6 µm. The integrated optics consists of a compound lens array with parallel light beam propagation within the tube path and were tailored to fit into the restricted space blow the MyoRobot rack. A rail guidance system, designed by our own workshop, allows precise component positioning and is part of a modular setup that can be expanded to include fluorescence imaging.
MyoRobot optics system featuring a basic compound lens alignment,allowing simple operation, while ensuring sufficient resolution to visualize sarcomeres.
Custom-written camera software (LabVIEW) allows edge (and thus fiber diameter) detection which is used to calculate cross-sectional area and specific force on-the-fly. Sarcomere length is determined via two methods (‘rising edge of derivative’ and ‘FFT of derivative’) that utilize the intensity line profile of the muscle fiber. All detection operations can be adjusted to the sample and current illumination conditions within the MyoWizzard environment. Ultimately, a quality criterion is displayed on the screen to facilitate the ease of use.
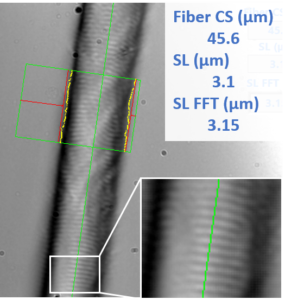 Picture of a recorded single muscle fiber within the MyoRobot system displaying a contrast-rich sarcomere pattern. Values obtained with the MyoWizzard are given in the top right corner.
Picture of a recorded single muscle fiber within the MyoRobot system displaying a contrast-rich sarcomere pattern. Values obtained with the MyoWizzard are given in the top right corner.